Electric Forces and Coulomb's Law
Tutorials on electric forces with examples and explanations.
Electric Forces
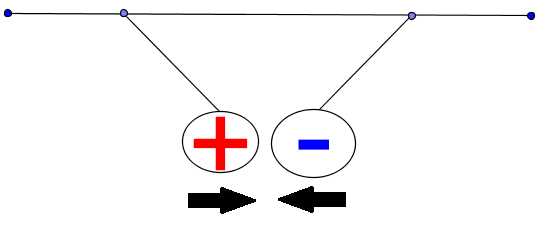
Electric charges of the same sign repel each other and charges of opposite signs attract each other.
Charges of opposite signs
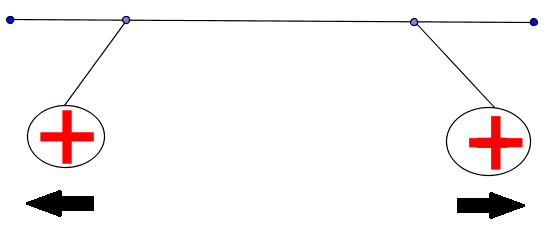
Both charges are positive
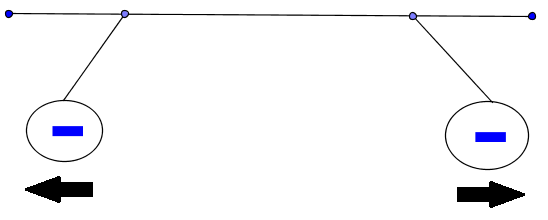
Both charges are negative
Coulomb's Law
The forces on two charges of opposite signs for example, separated by a distance r are quantified by Coulomb's law as follows:
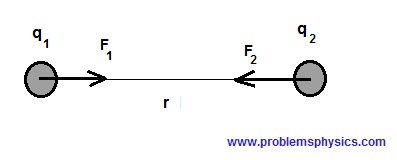
$|F_1| = |F_2| = k \dfrac{|q_1 q_2|}{r^2}$
where $|F_1|$ and $|F_2|$ are the magnitudes of forces $F_1$ and $F_2$, $r$ is the distance separating the two charges $q_1$ and $q_2$ and $k$ is a constant.
More Than Two Charges
In the example below, there are more than two charges: $q_1$ positive and $q_2$ and q3 negative. There are two forces acting on q1 and the resultant force is the sum of the forces $F_1$ + $F_3$ (addition of vectors since the forces are represented by vectors)




